HOW DID IT ALL START?

It started with our fascination with what everything is made up of. Democritus and Leucippus, 2 Greek guys though that everything may not be Greek, and proposed that particles are made of particles. Cool theory! The main point here was that this order of divisibility eventually terminates. They used to think that sand’which’ particles weren’t part of the dungeon of a ‘witch’, but were sandwich particles, much like how, very ironically, iron is made up of iron particles. There definitely is some irony to this.
VS HOW WAS IT GOING?

Scientists, much like us, have an affinity for neon signs, and therefore, a chemist named J.J. Thompson carried out the discharge tube experiment, in which he took a tube and passed electricity to it. This tube was evacuated (due to an obvious emergency!) and we loved it! We saw glowing light! This means that there exist electrons! Yesn’t. To confirm the existence of electrons, J.J. carried out the experiment with the help of several different gases, and observed that the particles had the same charge/mass ratio. He concluded that electrons were fundamental particles. Stoney, who definitely wasn’t made up of stone, named them ‘electrons’.
Similarly, Goldstein discovered protons. Neutrons, however, were discovered in a different way. Chadwick shot some bullets into the nucleus of an alpha particle, releasing neutrons in the process. This accounted for the difference in masses of an atom and the protons in an atom.
Rutherford performed his gold foil experiment in which he shot some alpha particles into an extremely thin gold foil. He observed that most of the particles went straight through the foil, some got deflected and very few of them suffered large deviations. He therefore concluded that electrons revolve around protons in circular orbits.
Also, in Rutherford’s experiment, Rutherford calculated the probability of an alpha particle retracing its path going straight through or being deflected. This is equal to the net area of the “nuclei” over the total area of the gold foil, therefore enabling him to calculate the radius of the Gold atom.
After this, we applied Newton’s laws to explain all this, which was found to be incompatible with our observations. (The observation here was that we are alive and atomic. Why? Because all this can’t be applied to the teeny tiny atoms.
Now comes the role of Bohr and Heisenberg. Bohr proposed that electrons revolved around the nucleus in planet-like orbits, in accordance with Rutherford’s experiment. He did some cool-looking math and then passed away. Heisenberg passed away too, after de Broglie and him destroyed the careers of other scientists before them by introducing the wave nature of particles (de Broglie) and proving that the exact position and velocity of a particle can’t be determined exactly (Heisenberg).
Goldstein suffered from the same fate. J.J. Thompson couldn’t escape his fate, Stoney passed away and Rutherford passed away. This means that everyone who did something in chemistry passed away. The same is true for all other subjects.
MODERN ATOMIC THEORY AND THE QUANTUM THEORY
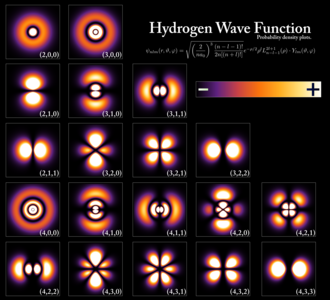
Quantum theory basically states that everything cannot be determined, and therefore has to be expressed in terms of probabilities. It has been successfully applied to microscopic objects (atoms and others), but doesn’t hold good for macroscopic forces at the large scale. It has been used by us to find the probability of finding an electron in an atom in a 3D space (and other subatomic particles).
At its heart is the cool-looking Schrodinger equation, which is a postulate proposed by Erwin Schrödinger, who assumed that electrons were waves capable of influencing their surroundings. It allows us to calculate the energy levels of an atom, which can help us to determine the structure of the atom. It can only be solved approximately for other atoms, but can be solved exactly for the Hydrogen atom.
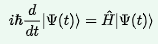
Here, t is time, i is the imaginary unit (defined as sqrt(-1)), H with a hat is the Hamiltonian operator, which corresponds to energy (Potential Energy + Kinetic Energy), and psi is the wave function, which is interpreted as the probability density (probability of finding an electron = psi^2). Also h bar (a cool looking h with a strike on it), is the reduced Planck’s constant.
Planck proposed his Quantum theory of radiation which said that energy is made up of discrete packets. This was the foundation for quantum mechanics.
Orbitals are places where you expect an electron to be (regions of high probability density). It comes in different sizes and shapes. For example, the s orbital is spherical and small, the p orbital is dumbbell shaped, and the d orbital is clove shaped (4 leaf). The f orbital contains a combination of 2 different orbitals (more like a double dumbbell)
These are the shapes of different orbitals:-
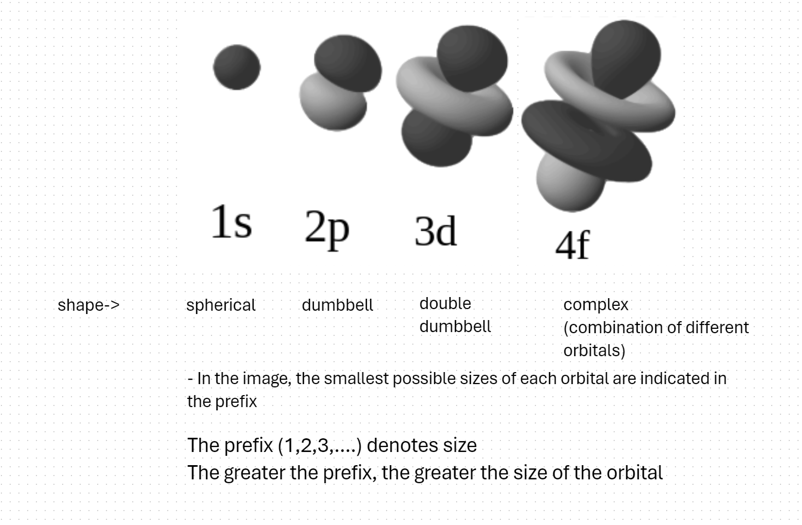
And that’s it. The cloud model successfully predicts regions where an electron is most likely to be found.
NUCLEAR CHEMISTRY AND THE STRUCTURE OF THE NUCLEUS
When we calculated the mass of the nucleus of a Helium atom, and compared it with the mass of 2 protons and 2 neutrons, we found a difference between the mass sums. This means that some mass of the nucleus is converted into extra energy (known as Binding energy) in accordance with the equation E=mc^2. This energy keeps the nucleus together.
The strong nuclear force is what wins over the coulombic repulsion (due to like charges) between the protons of a nucleus, causing the protons and neutrons to be “glued” together by the higgs boson, a fundamental particle, which is massless and chargeless. The strong nuclear force accounts for most of the mass of the protons and neutrons, and not the quarks of which they are made of.
Binding energy is the energy which is needed to keep a nucleus from separating into protons and neutrons, and is the highest for Iron.
The GUT, or the Grand Unified Theory, aims to bind together the aspects of the nuclear force with the electromagnetic force and weak force. This is in accordance with our assumption that the dawn of the universe saw the production of these forces from a single structure/entity. We don’t know what that is though. If we manage to draw out a Grand Unified Interaction which can explain both strong and weak interactions, we’ll be able to predict the behaviour of almost everything, unless we have access to an open source classified file describing everything in the universe which states that there’s more to be discovered.
The Standard Model is our best attempt at this, though it can’t explain the same magnitude of charges on the electron and proton.
The GUT has been built into some blocks or parts by the Electroweak theory, which tells us how the electromagnetic force and the weak nuclear force were the same in the early universe. This theory was majorly built by Maxwell’s equations, which describe the behaviour of electric and magnetic fields.
We can do this……
HOW DO WE REPRESENT ELEMENTS USING OUR NEWFOUND KNOWLEDGE?
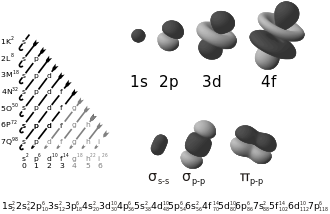
We represent elements on the periodic table via their electronic configuration, which is just the distribution of electrons in an atom according to the various orbitals in it. We do this to organise our work. If we can get a table through which we can instantly get to know about the positions of the electrons of the atom, we can get to know a lot about them.
This table is the Periodic Table (shown below):-
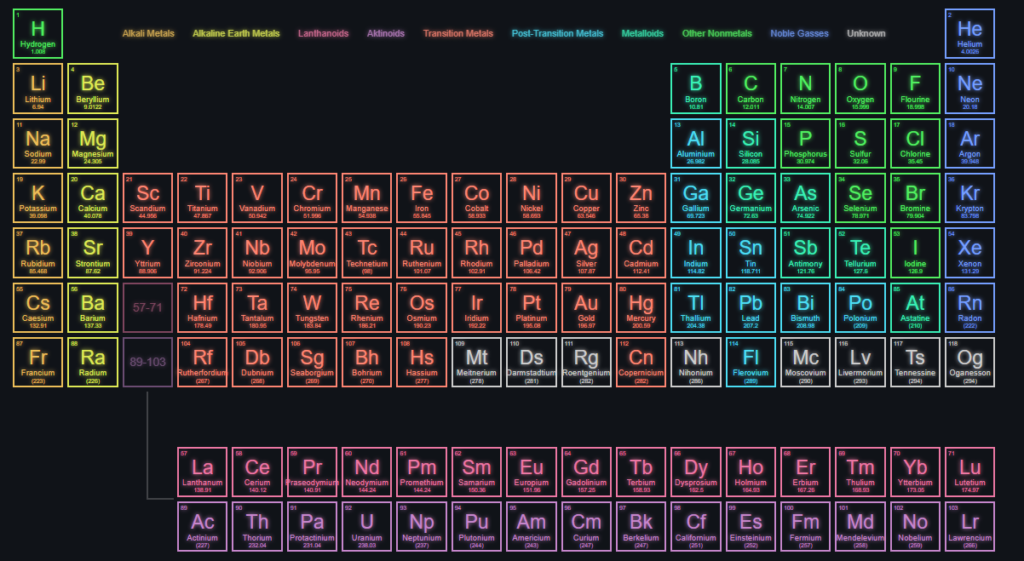
Wait but what does this s,p,d,f mean? Does it relate to orbitals? Yes. The elements whose electronic configuration ends with electron(s) in the s orbital are s block elements, the elements whose electronic configuration ends with electron(s) in the p orbital are p block elements, and so on..
But is there any correlation between the orbitals of an atom and its electronic configuration? There certainly is. This is because electrons are located in shells/orbits, which contains subshells, and further divisions which are attributed to quantum numbers (Principal, Azimuthal, Magnetic and Spin). Hydrogen’s electrons (if they gain energy and go to different orbits or if they don’t do so) can be completely described via these quantum numbers, but such a description isn’t possible for multielectronic atoms due to electron-electron interactions.
Therefore, we use orbitals to describe the electrons in an atom.
For example, consider the following electronic configuration process for Flerovium (Atomic Number= 114).
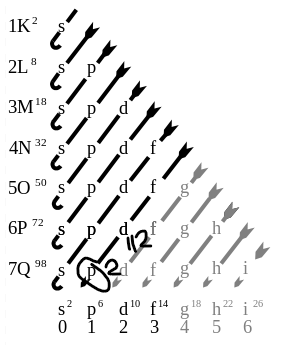
Here, the s orbital can house 2 electrons, the p orbital can have 6 electrons, the d electron can possess 10, and so on….
The orbitals with same energy are referred to as Degenerate Orbitals.
Therefore, the electronic configuration (full form) of Flerovium is the following:-
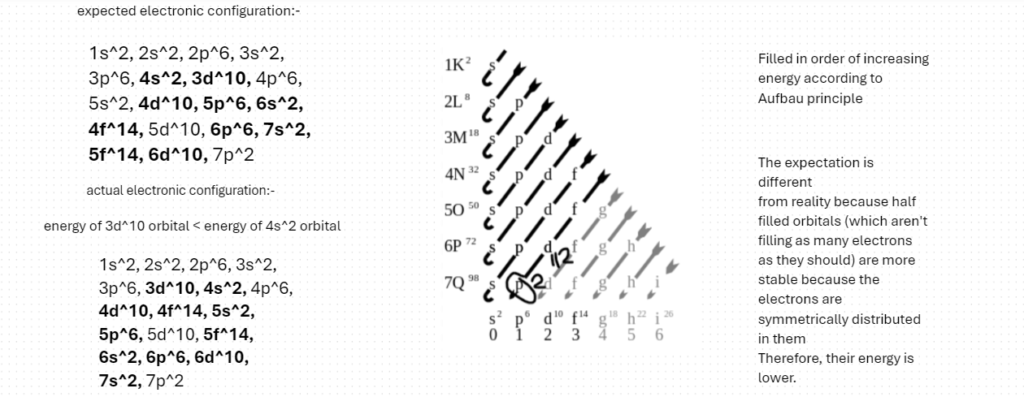
These differences also occur because the difference in the energies of the bolded orbitals are is very small, but we have to take that into consideration as well, because, after all, Reality is not bound by expectations. It can be whatever it wants to be.